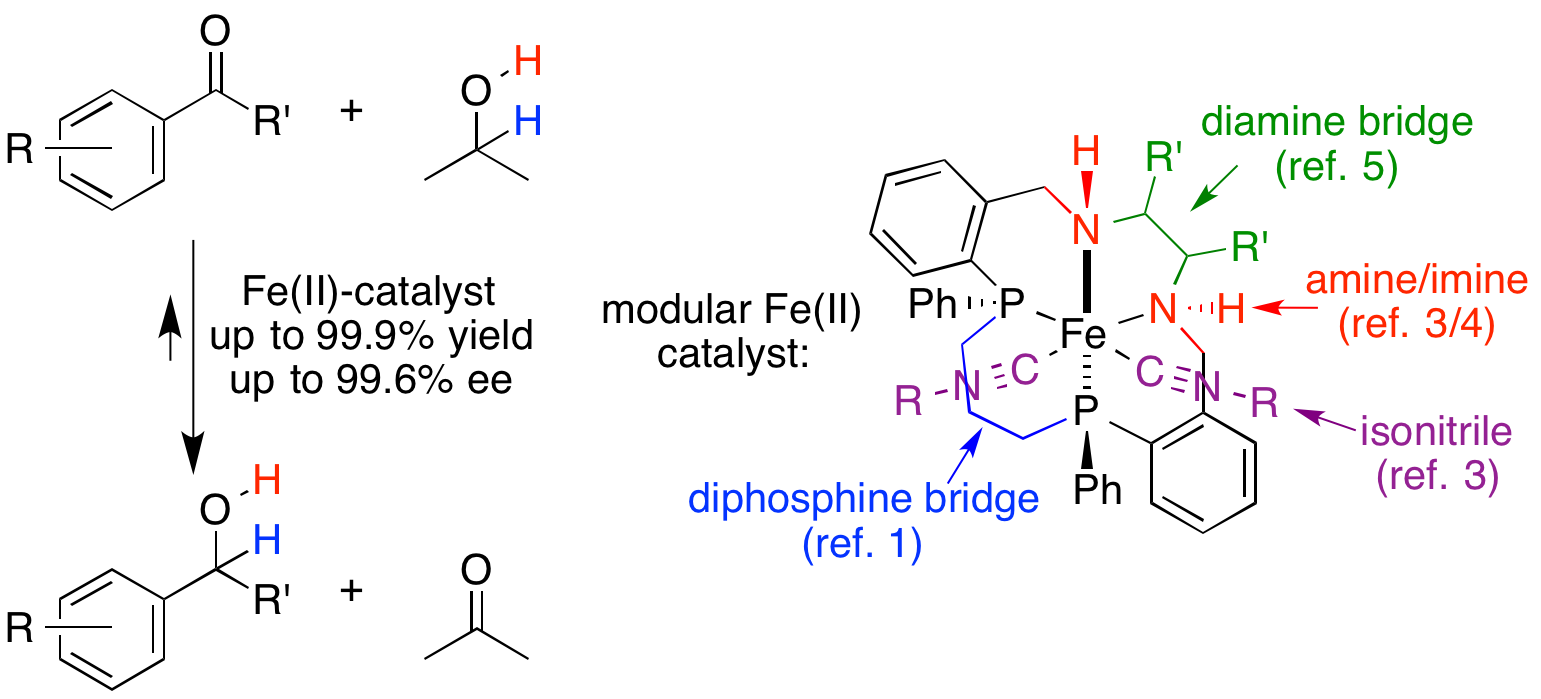
Asymmetric Transfer Hydrogenation with Bis(isonitrile) Complexes
A modular, scalable synthesis of macrocyclic N2P2 ligands allows the preparation of a range of stable, diamagnetic iron catalysts [Fe(CNR)2(N2P2)]2+ that can be further tuned by modifying the ancillary isonitrile ligands. These complexes are highly active and enantioselective catalysts in the transfer hydrogenation of polar double bonds and achieve up to 99.9% yield and 99.6% ee. The tunability of these catalysts makes the optimization for any desired substrate fast and straightforward.
References
1) "Asymmetric Transfer Hydrogenation with a Bifunctional Iron(II) Hydride: Experiment Meets Computation", L. De Luca, A. Passera, A. Mezzetti, J. Am. Chem. Soc. 2019, 141, 2545. external page DOI: 10.1021/jacs.8b12506.
2) "Iron Complexes with Chiral N/P Macrocycles as Catalysts for Asymmetric Transfer Hydrogenation", A. Mezzetti, Isr. J. Chem. 2017, 57, 1090. external page DOI: 10.1002/ijch.201700035.
3) "Iron(II)/(NH)2P2 Macrocycles: Modular, Highly Enantioselective Transfer Hydrogenation Catalysts", R. Bigler, R. Huber, M. Stöckli, A. Mezzetti, ACS Catal. 2016, 6, 6455. external page DOI: 10.1021/acscatal.6b01872.
4) "Highly Enantioselective Transfer Hydrogenation of Polar Double Bonds by Macrocyclic Iron(II) / (NH)2P2 Catalysts", R. Bigler, A. Mezzetti, Org. Process Res. Dev. 2016, 20, 253. external page DOI: 10.1021/acs.oprd.5b00391. This article was recapped in Chemical & Engineering News.
5) "Highly Enantioselective Transfer Hydrogenation of Ketones with Chiral (NH)2P2 Macrocyclic Iron(II) Complexes" R. Bigler, R. Huber, A. Mezzetti, Angew. Chem. Int. Ed., 2015, 54, 5171. external page DOI: 10.1002/anie.201501807.
6) "Isonitrile Iron(II) Complexes with Chiral N2P2 Macrocycles in the Enantioselective Transfer Hydrogenation of Ketones" R. Bigler, A. Mezzetti, Org. Lett., 2014, 16, 6460. external page DOI: 10.1021/ol503295c.
7) "Chiral Macrocyclic N2P2 Ligands and Iron(II): A Marriage of Interest" R. Bigler, E. Otth, A. Mezzetti; Organometallics, 2014, 33, 4086. external page DOI: 10.1021/om5005989