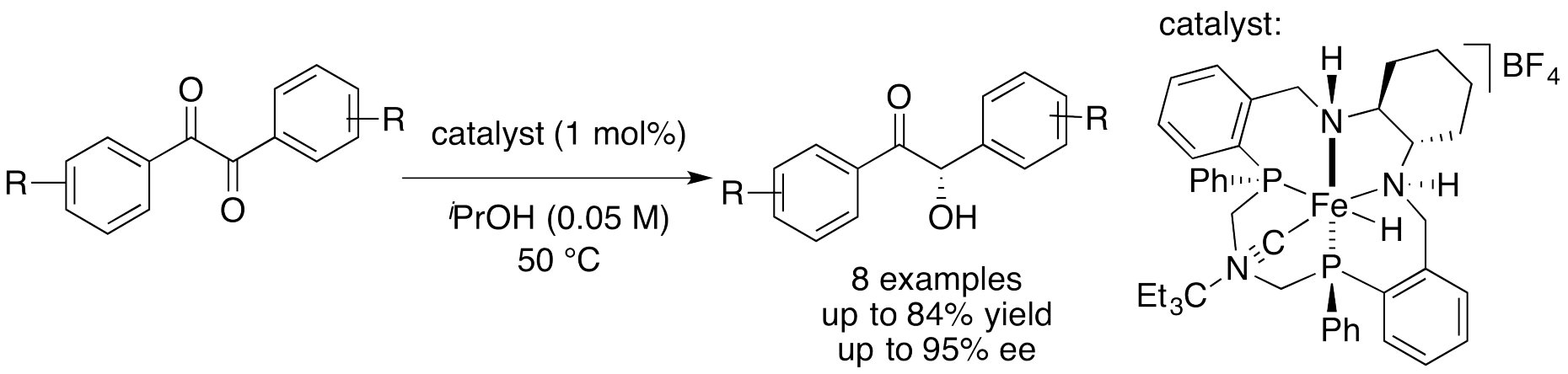
Base-free Monoreduction of Benzils with Fe(II) Hydrides
The chiral (NH)2P2 macrocyclic ligands developed by our group was used for the preparation of an iron(II) hydride that is active in the asymmetric transfer hydrogenation (ATH) of ketones without basic activation. The base-free conditions under which the catalyst operates enabled the preparation of chiral base-labile benzoins, in the first example of highly enantioselective hemiredution of cheap and readily available benzils.
References
1) "Asymmetric Transfer Hydrogenation with a Bifunctional Iron(II) Hydride: Experiment Meets Computation", L. De Luca, A. Passera, A. Mezzetti, J. Am. Chem. Soc. 2019, 141, 2545. external page DOI: 10.1021/jacs.8b12506.
2) "Base-free Asymmetric Transfer Hydrogenation of 1,2-Di- and Monoketones Catalyzed by a Chiral Iron(II) Hydride", L. De Luca, A. Mezzetti, CHIMIA 2018, 72, 233. external page DOI: 10.2533/chimia.2018.233.
3) "Base-Free Asymmetric Transfer Hydrogenation of 1,2-Di- and Monoketones Catalyzed by a (NH)2P2-Macrocyclic Iron(II) Hydride", L. De Luca, A. Mezzetti, Angew. Chem. Int. Ed. 2017, 56, 11949. external page DOI: 10.1002/anie.201706261.