Fe/PN(H)P Complexes
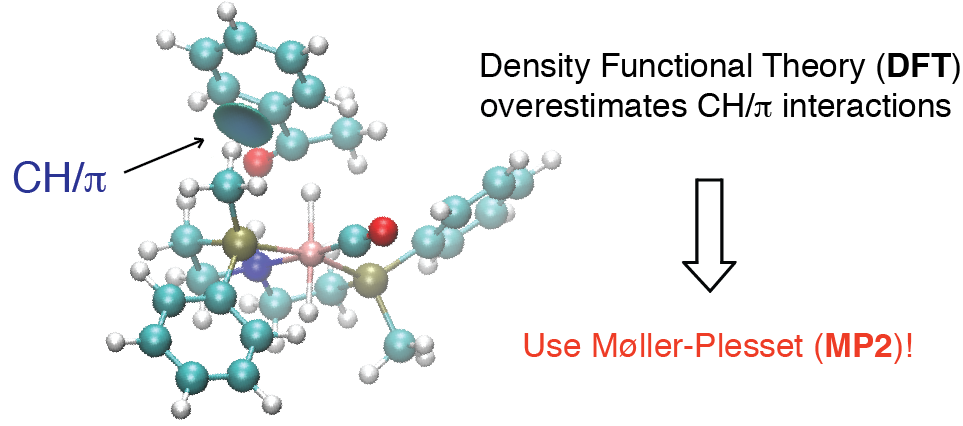
The P-stereogenic PN(H)P pincer ligands (R(Me)PCH2CH2)2NH (R = Cy, tBu or Ph) and their iron(II) derivatives [FeBr2(CO)(PN(H)P)] and [FeBrH(CO)(PN(H)P)] were developed by DFT-driven ligand design and tested in the asymmetric hydrogenation of ketones. A DFT study suggested that the Ph(Me)P-derived pincer should give high enantioselectivity, but in practice, 44% ee was achieved for acetophenone. The origin of this discrepancy was identified to be the incorrect assessment of CH/π interactions by DFT. Increasing the level of theory to post-Hartree-fock MP2 gives excellent agreement with experiment and is thus recommended for calculations in asymmetric catalysis where CH/π interactions are at the origin of enantioselectivity.
References
1) R. Huber, A. Passera, A. Mezzetti, ChemComm 2019, 55, 9251. external page DOI:10.1039/C9CC03910D.
2) R. Huber, A. Passera, E. Gubler, A. Mezzetti, Adv. Synth. Catal. 2018, 360, 2900. external page DOI: 10.1002/adsc.201800433.